All You Need to Know About Buckminsterfullerene!
By Emma Schwindt
Search This Blog
Bibliography
Aliexpress.com : Buy Free Shipping 5mm 216Pcs Buckyballs Magnetic Balls Sphere Cube Beads Neocube Magic Neodymium Cube Toys Solid Black from Reliable toys with free shipping suppliers on QINGYUAN OUTLETS | Alibaba Group. (n.d.). Retrieved May 5, 2015, from http://www.aliexpress.com/store/product/Free-Shipping-5mm-216Pcs-Buckyballs-Magnet-Balls-Cube-Beads-Neocube-Magic-Neodymium-Cube-Toys-Pure-Black/607429_669769474.html
Allotropes of carbon. (n.d.). Retrieved May 5, 2015, from http://afrodita.rcub.bg.ac.rs/~rzoran/Allotropes of carbon.htm
American, I. (1991). Scientific American triumph of discovery: A chronicle of great adventures in science (pp. 54-63). New York: H. Holt.
Buckminsterfullerene – A Review Covering The Discovery, Structure, Production, Properties and Applications of Buckyballs. (2006, July 15). Retrieved May 5, 2015, from http://www.azonano.com/article.aspx?ArticleID=1641
Buckyball Uses and Applications| Fullerene Applications. (2007). Retrieved May 5, 2015, from http://www.understandingnano.com/buckyballs-fullerenes.html
Chemguide: CIE A level chemistry support: Learning outcome 11.3(e). (n.d.). Retrieved May 5, 2015, from http://www.chemguide.co.uk/CIE/section113/learninge.html
Chemical Bonding. (n.d.). Retrieved May 5, 2015. http://www.users.csbsju.edu/~frioux/c60/BondingC60.pdf
Fellowship Program. (2012, February 13). Retrieved May 5, 2015, from http://c7group.com/about-us/careers/fellowship-program/
France, C. (2014). GCSE CHEMISTRY - What is Buckminsterfullerene? - What is the Structure of Buckminsterfullerene? - What is C60? - GCSE SCIENCE. Retrieved April 27, 2015, from http://www.gcsescience.com/a38-buckminsterfullerene.htm
Fullerene C60. (2014). Retrieved May 5, 2015, from http://www.alzdiscovery.org/cognitive-vitality/report/fullerene-c60
How to Draw a Soccer Ball. (n.d.). Retrieved April 27, 2015, from https://www.pinterest.com/pin/353603008217491383/
Richard E. Smalley, Buckminsterfullerene (the Buckyball), and Nanotubes. (n.d.). Retrieved May 5, 2015, from http://www.osti.gov/accomplishments/smalley.html
Science Clarified. (n.d.). Retrieved May 5, 2015, from http://www.scienceclarified.com/Di-El/Electric-Arc.html
Surface tension of C60 solutions in toluene. (n.d.). Retrieved May 5, 2015, from http://adsabs.harvard.edu/abs/1993JChPh..98.7648X
Trend in electrical conductivity of Period 3 elements. (2014, February 19). Retrieved May 5, 2015, from http://www.creative-chemistry.org.uk/alevel/module1/trends9.htm
Allotropes of carbon. (n.d.). Retrieved May 5, 2015, from http://afrodita.rcub.bg.ac.rs/~rzoran/Allotropes of carbon.htm
American, I. (1991). Scientific American triumph of discovery: A chronicle of great adventures in science (pp. 54-63). New York: H. Holt.
Buckminsterfullerene – A Review Covering The Discovery, Structure, Production, Properties and Applications of Buckyballs. (2006, July 15). Retrieved May 5, 2015, from http://www.azonano.com/article.aspx?ArticleID=1641
Buckyball Uses and Applications| Fullerene Applications. (2007). Retrieved May 5, 2015, from http://www.understandingnano.com/buckyballs-fullerenes.html
Chemguide: CIE A level chemistry support: Learning outcome 11.3(e). (n.d.). Retrieved May 5, 2015, from http://www.chemguide.co.uk/CIE/section113/learninge.html
Chemical Bonding. (n.d.). Retrieved May 5, 2015. http://www.users.csbsju.edu/~frioux/c60/BondingC60.pdf
Fellowship Program. (2012, February 13). Retrieved May 5, 2015, from http://c7group.com/about-us/careers/fellowship-program/
France, C. (2014). GCSE CHEMISTRY - What is Buckminsterfullerene? - What is the Structure of Buckminsterfullerene? - What is C60? - GCSE SCIENCE. Retrieved April 27, 2015, from http://www.gcsescience.com/a38-buckminsterfullerene.htm
Fullerene C60. (2014). Retrieved May 5, 2015, from http://www.alzdiscovery.org/cognitive-vitality/report/fullerene-c60
How to Draw a Soccer Ball. (n.d.). Retrieved April 27, 2015, from https://www.pinterest.com/pin/353603008217491383/
Richard E. Smalley, Buckminsterfullerene (the Buckyball), and Nanotubes. (n.d.). Retrieved May 5, 2015, from http://www.osti.gov/accomplishments/smalley.html
Science Clarified. (n.d.). Retrieved May 5, 2015, from http://www.scienceclarified.com/Di-El/Electric-Arc.html
Surface tension of C60 solutions in toluene. (n.d.). Retrieved May 5, 2015, from http://adsabs.harvard.edu/abs/1993JChPh..98.7648X
Trend in electrical conductivity of Period 3 elements. (2014, February 19). Retrieved May 5, 2015, from http://www.creative-chemistry.org.uk/alevel/module1/trends9.htm
Observed relationships between types of intermolecular bonding/polarity.
Since it's known that two non-metals make covalent bonds, and carbon is a non-metal, the only question is whether or not two carbons make a polar or non-polar bond? Well, looking at the fact that their electronegativity values are the same, that would mean that buckminsterfullerene (C60) is a non-polar molecule. With this fact, we can also conclude that buckyballs must be symmetrical.
In terms of intermolecular bonding present in buckminsterfullerene, in the solid is Van Der Waals dispersion forces. This directly links with the fact that it has a lower boiling point than the other two common allotropes of carbon, diamond and graphite, because they would have a lot more covalent bonding going on (Chemguide, n.d.).
Then just to finish off the observations about whats going on inside this molecule, theres also some hybridization going on. Following the VSEPR theory, each carbon has sp^2 hybridization with the remaining p-orbital available for bonding with one of the adjacent carbon atoms. So overall, each carbon forms three bonds with its sp^2 hybrid orbital and one bond with the remaining p-orbital (Chemical Bonding, n.d.).
Because I knew you must have been curious... Can buckyballs have a Lewis structure?
The best way to answer the question, "Can buckyballs have a Lewis structure?" is by saying that it is an allotrope of carbon. Allotropes are different physical forms of the same element. In this case, buckminsterfullerene cannot have a Lewis structure, or at least that's not the best way to show the configuration of buckyballs. In figure 1, you can see there is a Lewis structure for just one carbon atom all alone, with its four valence electrons. The best way to represent this certain allotrope of carbon (C60) is by drawing out its soccer ball, spherical shape, indicating that the blue circles are the carbon atoms, every yellow line shows a double bond, and every red line shows a single bond (as seen in figure 2). Since we know, looking once again at figure 1, that every carbon atom has four valence electrons therefore making four bonds. Other allotropes of carbon - such as diamond and graphite - would be the same, it's not ideal to represent them by trying to draw a Lewis structure.
| Figure 2 - Buckminsterfullerene |
 |
| Figure 1 - Carbon Lewis Structure |
Real world applications in medicine, science, and industry.
Buckminsterfullerene has a lot of real world applications, and the first area of discussion is in medicine. Buckyballs are used in medicine as drug delivery systems into the body, pharmaceuticals, antioxidants, optical devices and even targeted cancer therapies (Azonano, 2006). The antioxidant properties of buckyballs might even be able to fight the deterioration of motor function due to multiple sclerosis!
The fact that they can be used as drug delivery systems into the body also leads into the next category, how buckyballs are used in science. The ways that the buckyballs can be used to deliver drugs into the body also act as two big ways it's used in science, in lubricants and as catalysts. Buckyballs are catalysts because of their high reactivity. Other ways it is used in science is as superconductors, chemical sensors and polymer additives (Azonano, 2006). Buckyballs may even -and are currently being tested - be able to reduce the growth of bacteria in pipes and membranes in water systems.
Then the lastly, how are buckyballs being used in industry? Well, the biggest thing with every industry right now is - aside from making the most money - 'how can we be green?' And what better way of going green is there than the fuel cell powered cars? Plus buckminsterfullerene can help with that. Buckyballs have such a large hollow structure that they can actually store hydrogen! And this hydrogen storage can be used as a fuel tank for fuel cell powered cars (Understanding Nano, 2007).
Overall, inventors have definitely used specific characteristics such as its structure, high reactivity, antioxidant properties and ability to have a modified shape to benefit society. The modified shape ability is really beneficial to society because researchers are currently attempting to modify buckyballs to fit the section of the HIV molecule that binds to proteins, possibly inhibiting the spread of the virus (Understanding Nano, 2007)!
The fact that they can be used as drug delivery systems into the body also leads into the next category, how buckyballs are used in science. The ways that the buckyballs can be used to deliver drugs into the body also act as two big ways it's used in science, in lubricants and as catalysts. Buckyballs are catalysts because of their high reactivity. Other ways it is used in science is as superconductors, chemical sensors and polymer additives (Azonano, 2006). Buckyballs may even -and are currently being tested - be able to reduce the growth of bacteria in pipes and membranes in water systems.
Then the lastly, how are buckyballs being used in industry? Well, the biggest thing with every industry right now is - aside from making the most money - 'how can we be green?' And what better way of going green is there than the fuel cell powered cars? Plus buckminsterfullerene can help with that. Buckyballs have such a large hollow structure that they can actually store hydrogen! And this hydrogen storage can be used as a fuel tank for fuel cell powered cars (Understanding Nano, 2007).
Overall, inventors have definitely used specific characteristics such as its structure, high reactivity, antioxidant properties and ability to have a modified shape to benefit society. The modified shape ability is really beneficial to society because researchers are currently attempting to modify buckyballs to fit the section of the HIV molecule that binds to proteins, possibly inhibiting the spread of the virus (Understanding Nano, 2007)!
What is the chemical composition of buckminsterfullerene?
As mentioned in the first post, buckminsterfullerene has a really unique structure and its even been said that it's the 'roundest molecule that can possibly exist' (Scientific American, 1991, pg. 54-63).
The simplest way to describe the structure of buckminsterfullerene is by comparing it to a soccer ball - which is a truncated icosahedron - and placing a carbon at the corner of every one pentagon and two hexagons until 60 carbons has been reached. In figure 1, all the dark blue spheres represent how the arrangement of carbon appears in buckyballs.
Buckminsterfullerene is a natural substance, found in soot and ash (Alzheimer's Drug Discovery Foundation, 2014). And although fullerenes are found in things as simple as candle soot, the most common technique for the production of fullerenes isn't quite that simple. It involves the "establishment of an electric arc between two carbon electrodes. Under these conditions, the energy from the arc is dissipated by breaking carbon from the surface. The carbon cools in the inert atmosphere and forms buckyballs" (Azonano, 2006). Figure 2 shows what the electric current (flow of electrons) would look like between an electric arc, similar to the electric arc that has to occur between two carbon electrodes for the production of buckyballs.
 |
| Figure 1 - Buckminsterfullerene |
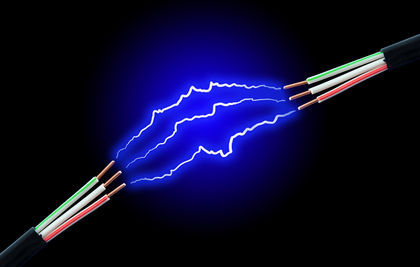 |
| Figure 2 - Electric Current from Electric Arc |
Wondering about the physical properties of buckminsterfullerene? Look no further.
The first physical property to discuss of buckminsterfullerene is the density. It has a density of 1.65 g/cm-3, and that number shows the relationship between the mass of the substance and the amount of space it takes up (volume). The second physical property is standard heat of formation, which is 9.08 k/cal/mol-1. This property has to do with enthalpy changes. The third physical property is thermal conductivity, which is 0.4W/mK. This has to do with buckyballs ability to conduct heat. Fourth, its boiling point is that it sublimes at 800K, which means it goes directly from a solid to a gas and doesn't pass through the liquid stage. Then lastly its melting point is that is sublimates at 843K (Kumar, n.d.).
When other physical properties that couldn't be described by exact numbers were tested, these were the results. To start off, buckminsterfullerene was found to be surprisingly surface active, occupying nearly a monolayer in a study done (SAO/NASA ADS, n.d.). Proving the fact that buckyballs have strong surface tension. Electrical conductivity was found to be very high, mainly because, like graphite, it has delocalized electrons. Those electrons are free to move and carry charge (Creative Chemistry, 2014). And buckminsterfullerene was found to actually be totally insoluble in water, but soluble in many common solvents such as benzene, toluene and chloroform (Kumar, n.d.).
Buckyballs appear as an odourless, black solid and have a sort of metallic lustre. In figure 1 there is a photograph of what buckyballs look like in the form of magnets. There hardness was unknown, but when compressed to 70% of its initial volume, its expected to become harder than a diamond (Allotropes of Carbon, n.d.).
When other physical properties that couldn't be described by exact numbers were tested, these were the results. To start off, buckminsterfullerene was found to be surprisingly surface active, occupying nearly a monolayer in a study done (SAO/NASA ADS, n.d.). Proving the fact that buckyballs have strong surface tension. Electrical conductivity was found to be very high, mainly because, like graphite, it has delocalized electrons. Those electrons are free to move and carry charge (Creative Chemistry, 2014). And buckminsterfullerene was found to actually be totally insoluble in water, but soluble in many common solvents such as benzene, toluene and chloroform (Kumar, n.d.).
 |
| Figure 1 - Buckyball Magnets |
Buckyballs appear as an odourless, black solid and have a sort of metallic lustre. In figure 1 there is a photograph of what buckyballs look like in the form of magnets. There hardness was unknown, but when compressed to 70% of its initial volume, its expected to become harder than a diamond (Allotropes of Carbon, n.d.).
What is buckminsterfullerene? (and where on earth did it get such a strange name?)
 |
| Figure 1 - Soccer Ball |
In 1985 scientists discovered that carbon (C60) had a new existing form. If you look at a soccer ball (see figure 1), it is composed of two shapes; hexagons and pentagons. This is similar to the structure of buckminsterfullerene. Sixty carbon atoms make up this spherical shape with a carbon
 |
| Figure 2 - Buckminsterfullerene |
To answer why it has such a long and unusual name there are two reasons, first, it is named after Richard Buckminster Fuller, an American architect who simplified the geodesic dome which links to the shape of buckyballs. And second, a 'fullerene' is just the name given to a molecule of carbon in either a hollow sphere, tube or many other different shapes.
Interestingly enough, since C60 was discovered there has since been many more carbon molecules made, there is now also C70, C76 and C84 (France, 2014).
Subscribe to:
Posts (Atom)