As mentioned in the first post, buckminsterfullerene has a really unique structure and its even been said that it's the 'roundest molecule that can possibly exist' (Scientific American, 1991, pg. 54-63).
 |
| Figure 1 - Buckminsterfullerene |
The simplest way to describe the structure of buckminsterfullerene is by comparing it to a soccer ball - which is a truncated icosahedron - and placing a carbon at the corner of every one pentagon and two hexagons until 60 carbons has been reached. In figure 1, all the dark blue spheres represent how the arrangement of carbon appears in buckyballs.
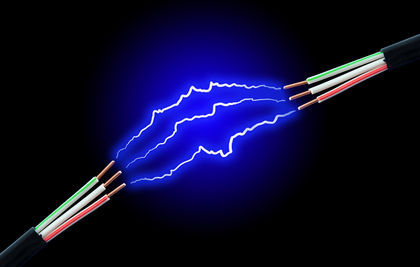 |
Figure 2 - Electric Current from
Electric Arc |
Buckminsterfullerene is a natural substance, found in soot and ash (Alzheimer's Drug Discovery Foundation, 2014). And although fullerenes are found in things as simple as candle soot, the most common technique for the production of fullerenes isn't quite that simple. It involves the "establishment of an electric arc between two carbon electrodes. Under these conditions, the energy from the arc is dissipated by breaking carbon from the surface. The carbon cools in the inert atmosphere and forms buckyballs" (Azonano, 2006). Figure 2 shows what the electric current (flow of electrons) would look like between an electric arc, similar to the electric arc that has to occur between two carbon electrodes for the production of buckyballs.



